
The Technology
The plant in Bakki is designed to produce ≥ 98.5% pure silicon metal from imported raw materials, that is, quartz and carbon; reactive coals with low ash content, wood chips, and a small amount of limestone.
The raw material will be moved by sea to Húsavík harbor and from there with special carriages to the raw material storage through a new industrial road and tunnel between the harbor and the plant. Thus, all material transport avoids the densely populated area of Húsavík.
In Phase 1, production will take place in two submerged arc furnaces. Each of them utilizes 24 MW of electrical power for the annual production of a total of approx. 32,000 metric tons of the final product, that is, silicon metal. For the production, we use pre-baked graphite electrodes.
In addition to the electricity used for the silicon metal production, 4 MW is needed to drive the gas cleaning plants (GCP) which treat the exhaust from the furnaces. The total power consumption of the plant in Phase 1, is therefore 52 MW. With the intended expansion, which involves the addition of two arc furnaces of the same size, the total power consumption of the plant increases to 104 MW.
The Production

Figure 1: A submerged arc furnace (image by SMS Siemag).
Most of the quartz (SiO2) is reduced by reactive coal (C) to silicon carbide (SiC), which then works as a reducing agent for the part of the quartz which has not yet reacted beneath the electrodes.
The overall chemical reaction in the production of silicon metal can be described with the following formula:
SiO₂ + 2C = Si + 2{CO}
SiO₂ + 3C = SiC + 2{CO} (at the bottom of the furnace)
These chemical reactions convert about 66% of the silicon and 100% of the carbon in the charge to silicon carbide (SiC). The final reaction takes place above the center of the furnace at a temperature higher than 1,835°C. The electric arc between the electrode ends and the center maintains a temperature of up to 2,000°C which is necessary for the final reaction of silicon carbide (SiC) and silicon dioxide (SiO2 and SiO) can take place:
2SiO₂ + 3SiC = 4Si + {SiO} +3{CO}
SiO + SiC = 2Si + {CO}
During this chemical reaction, the silicon in the chemical compounds SiO2, SiO and SiC are converted to pure silicon, which collects and is finally tapped from the furnace as silicon metal.
The overall chemical reaction is never absolutely complete, but silicon production can achieve up to 95% with optimum control of the processes. The right scaling and installation of furnaces, auxiliary and side equipment and skilled people in production control are essential for the utilization of the plant.
Figure 5 shows a typical submerged arc furnace, similar to the one that will be used in PCC’s plant.
The processes of silicon metal production are shown in Figure 2.
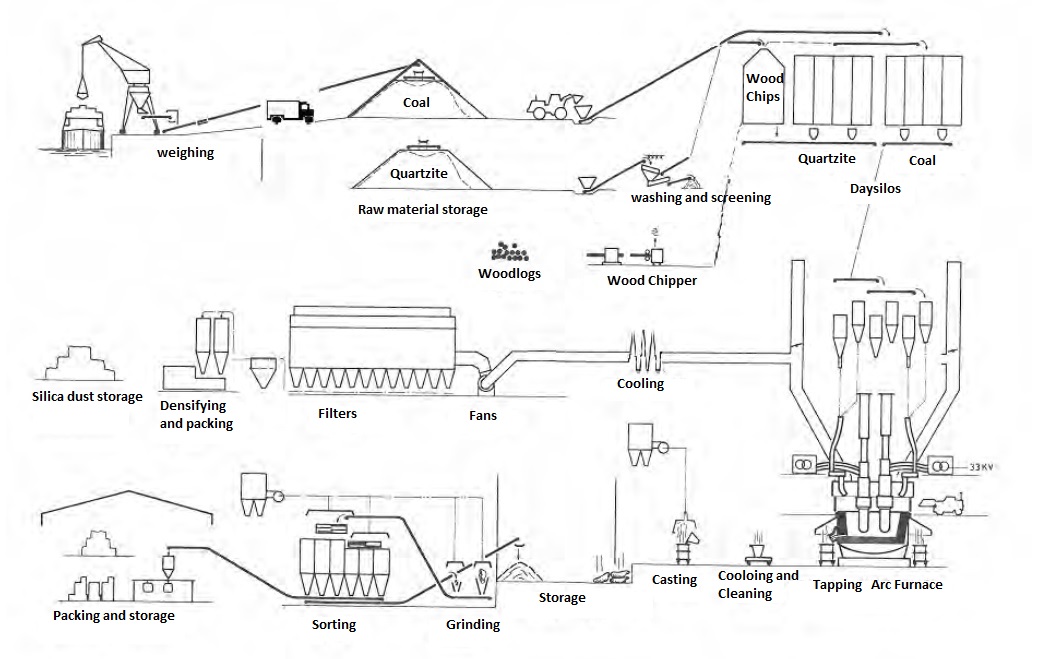
Figure 2: A simplified diagram of silicon metal production
The submerged arc furnace is divided into two parts: the upper portion above the smoke hood, and the lower portion, where the actual chemical reaction takes place and silicon metal is produced.
Pre-baked electrodes are located in the upper portion of the furnace and go through the smoke hood down to the lower portion, where the chemical reaction takes place. Exhaust gas is collected into the smoke hood and conducted to the gas cleaning plants (GCP) with bag filters. Heat is also conducted out of the hearth through the smoke hood.
Molten silicon metal is regularly tapped from the furnaces into crucibles and transferred to the casthouse where it is refined and cast into ingots. After cooling, the ingots are pre-crushed and stored temporarily within the furnace building. The product is later taken to crushing. After crushing the material is graded, packaged and transferred to the warehouse where it awaits sea transport.
The production process also generates MicroSilica, which is separated from the exhaust in bag filters in the GCP. During the production process, a very small amount of solid by-products are generated, as the majority of the raw materials is converted into silicon metal; a small part of them turns to slag. All exhaust from the furnaces and the crucible (i.e. the refining process) is treated.